|
|
|
|
|
|
|
|
|
|
|
|
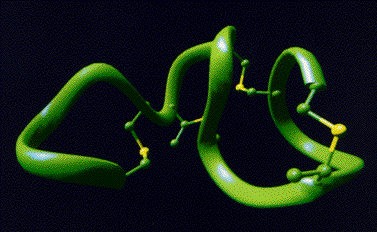 Microcins
and lantibiotics
are two rapidly
growing families of polypeptide antibiotics.
Both are synthesized ribosomally as precursor proteins, which undergo posttranslational
modifications of the amino acids serine, threonine and cysteine.
These modifications result in the formation of sulfide rings, 2,3-didehydroamino acids,
D-amino acids, oxazole and thiazole rings. Posttranslationally modified polypeptides are
limited in their conformational space.
Therefore a number of 3D structures were determined by multidimensional NMR.
In cooperation with the Department of Microbiology, the biosynthesis
is being investigated and new modifying enzymes are being characterized with respect to their structure and
specificity. For the first time, the construction of new modified peptides proved to be possible
by protein engineering. These techniques also provide the molecular tools for the development
of a new anti-acne drug as well as new gyrase inhibitors.
Microcins
and lantibiotics
are two rapidly
growing families of polypeptide antibiotics.
Both are synthesized ribosomally as precursor proteins, which undergo posttranslational
modifications of the amino acids serine, threonine and cysteine.
These modifications result in the formation of sulfide rings, 2,3-didehydroamino acids,
D-amino acids, oxazole and thiazole rings. Posttranslationally modified polypeptides are
limited in their conformational space.
Therefore a number of 3D structures were determined by multidimensional NMR.
In cooperation with the Department of Microbiology, the biosynthesis
is being investigated and new modifying enzymes are being characterized with respect to their structure and
specificity. For the first time, the construction of new modified peptides proved to be possible
by protein engineering. These techniques also provide the molecular tools for the development
of a new anti-acne drug as well as new gyrase inhibitors.
|

1. N. Schnell, K.-D- Entian, U. Schneider, F. Götz, H. Zähner, R. Kellner,
and G. Jung (1988).
Prepeptide Sequence of Epidermin, a Ribosomally Synthesized Polypeptide
Antibiotic Containing four Sulphide-Rings,
Nature 333, 276-278.
2. R. Kellner, G. Jung, M. Josten, C. Kaletta, K.-D. Entian, and H.-G. Sahl (1989).
Pep5: Strukturaufklärung eines großen Lantibioticums,
Angew. Chem. 101, 618-621; Angew. Chem. Int. Ed. Engl. 28, 616-619.
3. G. Jung (1991).
Lantibiotica, Ribosomal Synthetisierte Polypeptid-Wirkstoffe mit Sul-
fidbrücken und a,b-Didehydroaminosäuren,
Angew. Chem.
103, 1067-1084; Angew. Chem. Int. Ed. Engl. 30, 1051-1068.
4. G. Jung and H.-G. Sahl (1991) (eds.).
Nisin und Novel Lantibiotics,
490 pp., Escom, Leiden.
5. N. Zimmermann, S. Freund, A. Fredenhagen, and G. Jung (1993).
Solution Structures of the Lantibiotics Duramycin B and C,
Eur. J.
Biochem. 216, 419-428.
6. A. Bayer, S. Freund, G. Nicholson und G. Jung (1993).
Posttranslationale Backbone-Modifikation unter Bildung heteroaromatischer
Fünfringe bei der Biosynthese des glycinreichen Antibiotikums Microcin
B17,
Angew. Chem. 105, 1410-1413; Angew. Chem. Int. Ed. Engl.32, 1336-
1339.
7. M. Skaugen, J. Nissen-Meyer, G. Jung, S. Stevanovic´, K.Sletten, C.I. Mortvedt
Abildgaard, and I.F. Nes (1994).
In vivo
Conversion of L-Serine to D-Alanine in a Ribosomally Synthesized
Polypeptide,
J. Biol. Chem. 269, 27183-27185.
8. T. Kupke, C. Kempter, V. Gnau, G. Jung, and F. Götz (1994).
Mass Spectrometric Analysis of a Novel Enzymatic Reaction: Oxidative Decarboxylation
of the Lantibiotic Precursor Peptide EpiA Catalyzed by the Flavoprotein EpiD,
J. Biol. Chem. 269, 5653-5659.
9. R. Jack, A. Carne, J. Metzger, S. Freund, H.-G. Sahl, G. Jung, and J. Tagg
(1994).
Elucidation of the Primary Structure of SA-FF22, a Lanthionine-Containing
Antibacterial Peptide Produced by
Streptococcus pyogenes
,
Eur. J. Biochem. 220, 455-462.
10. A. Bayer, S. Freund, and G. Jung (1995).
Post-translational Heterocyclic Backbone Modifications in the 43-Peptide Antibiotic
Microcin B17, Structure Elucidation and NMR Study of a
13
C,
15
N-labelled Gyrase Inhibitor,
Eur. J. Biochem.234, 414-
426.
11. T. Kupke, C. Kempter, G. Jung, and F. Götz (1995).
Oxidative Decarboxylation of Peptides Catalyzed by Flavoprotein Epi D: Determination
of Substrate Specificity Using Peptide Libraries and Neutral Loss
Mass Spectrometry,
J. Biol. Chem. 270, 11282-11289.
12. B.Ottenwälder, T. Kupke, S. Brecht, V. Gnau, J. Metzger, G. Jung, and F.
Götz (1995).
Isolation and Characterization of Genetically Engineered Gallidermin and
Epidermin Analogs,
Appl. Environ. Microbiol. 61, 3894-3903.
13. O. Potterat, H. Stephan, J.W. Metzger, V. Gnau, H. Zähner, and G. Jung
(1994).
Aborycin - a Tricyclic 21-Peptide Antibiotic Isolated from
Streptomyces
griseoflavus,
Liebigs Ann. Chem. 1994, 741-743.
14. N. Zimmermann, J.W. Metzger, and G. Jung (1995).
The Tetracyclic Lantibiotic Actagardine,
1
H-NMR and
13
C-NMR Assignments and Revised Primary Structure,
Eur. J.
Biochem., 228, 786-797.
15. F.J.M. van de Ven and G. Jung (1996).
Structures of Lantibiotics Studied by NMR,
Anthonie van Leeuwenhoek
J. Microbiol. 69, 99-107.
16. O.P. Knipers, G. Bierbaum, B. Ottenwälder, H.M. Dodd, N. Horn, J.W. Metzger, T. Kupke,
V. Gnau, R. Bongers, P. van den Bogaard, K. Kosters, H.S. Rollema, W. M. de Vos, R.J. Siezen,
G. Jung, F. Götz, H.-G. Sahl, and M.J. Gasson (1996)
Protein Engineering of Lantibiotics,
Antonie van Leeuwenhoek J. Microbiol. 69, 161-170.
17. C. Kempter, T. Kupke, D. Kaiser, J.W. Metzger, and G. Jung (1996)
Thioenols from Peptidyl-cysteines: Oxidative Decarboxylation of a
13
C-Labeled Substrate,
Angew. Chem. 108, 2235-2238; Angew. Chem. Int. Ed. Engl. 35, 2104-2107.
18. G. Videnov, D. Kaiser, C. Kempter, M. Brooks, and G. Jung (1996)
Synthesis of the DNA Gyrase Inhibitor Microcin B17, a 43-Peptide Antibiotic with
Eight Heteroaromatic Rings in the Backbone,
Angew. Chem. 108,1607-1609; Angew. Chem. Int. Ed. Engl. 35,1506-1508.
19. G. Videnov, D. Kaiser, C. Kempter, and G. Jung (1996)
Synthesis of Naturally Occuring, Conformationally Restricted Oxazole and Thiazole Containing Di- and
Tripeptide Mimetics,
Angew. Chem. 108, 1604-1607; Angew. Chem. Int. Ed. Engl. 35, 1503-1506.
20. G. Bierbaum, C. Szekat, M. Josten, C. Heidrich, C. Kempter, G. Jung, and H.-G. Sahl (1996)
Engineering of a Novel Thioether Bridge and Role of Modified Residues in the LAntibiotic Pep5,
Appl. Environm. Microbiol. 62, 385-392.
21. G. Fimland, O.R. Blingsmo, K. Sletten, G. Jung, I.F. Nes, and J. Nissen-Meyer (1996)
New Biologically Active Hybrid Bacteriocins Constructed by Combining Regions from Various
Pediocin-like Bacteriocins: The C-Terminal Region is Important for Determining Specificity
Appl. Environm. Microbiol. 62, 3313-3318.
22. N. Zimmermann and G. Jung (1997)
The Three-dimensional Solution Structure of the Lantibiotic Murein-Biosynthesis Inhibitor Actagardine
Determined by NMR,
Eur. J. Biochem. 246, 809-819.
23. A.M. Israil, R. Jack, G. Jung, and H.-G. Sahl (1996)
Isolation of a New Epidermin Variant from two Strains of
Staphylococcus epidermidis
- Frequency
of Lantibiotic Production in Coagulase-negative Staphylococci
Zbl. Bakt. 284, 285-296.
24. R. Jack, F. Götz, and G. Jung (1997)
Lantibiotics,
in: H.-J. Rehm and G. Reed (eds.), Biotechnology, Vol. 7, Chapter 8, pp. 323-368, Verlag Chemie, Weinheim
